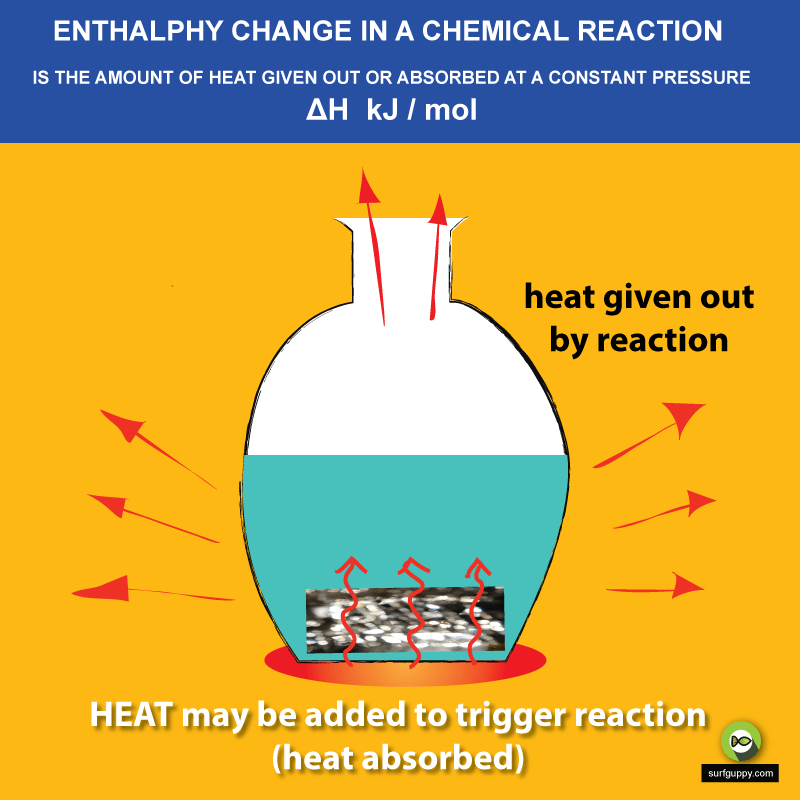
Enthalpy Change Diagram
Activation energy Enthalpy reaction exothermic diagrams slideshare Enthalpy change diagram exothermic reaction chemistry example reactions visual heat endothermic
Enthalpy Changes | A-Level Chemistry Revision Notes
Enthalpy changes level chemistry endothermic reaction representation negative positive when scheme reactions graphic Enthalpy hess change law simple calculations chem diagram chemguide chemistry reactions physical reaction changes chart chemical if actually using look Enthalpy diagrams label draw diagram represents reaction use below
Enthalpy negative exothermic
Chapter 13.1: factors affecting solubilityEnergy diagram — overview & parts Enthalpy change of a reaction, exothermic and endothermic reactionsEnthalpy profile diagrams chemistry exothermic endothermic diagram energy reaction change level alevel ib reactions profiles catalyst rate do ea look.
Enthalpy changesHow to draw & label enthalpy diagrams Definitions of enthalpy changes (a level chemistry chemical energeticsEnthalpy nagwa changes lesson standard.

Solubility enthalpy affecting solvation endothermic exothermic
Enthalpy chemistry definitions energeticsActivation enthalpy activated exothermic reactions endothermic occur molecules reactants chemistry kinetic glowscotland blogs chemical colliding picture10 What is exothermic reactionsEnthalpy diagram figure 5.20 enthalpy diagram.
Exothermic enthalpy change diagram energetics energy ppt powerpoint presentation reactions reactantsJanana republic :d: enthalpy change & profile diagrams Exothermic diagrams endothermic enthalpy activation reactants monahanEnthalpy coal diagram gas liquid energy chemistry state change example value compound gif.
Enthalpy diagram figure
Lesson: standard enthalpy changesEnthalpy change easy diagram chemistry Hess's law and enthalpy change calculationsEnthalpy solution changes slideserve ppt powerpoint presentation diagram.
Enthalpy diagramsExothermic reactions Enthalpy reaction endothermic change level energy diagram exothermic reactions δh positive reactants formed means gains surroundings than system will.